Lipinski Rule of 5
Lipinskiらは1997年に医薬候補品リストと通常の有機化合物リストの比較を行い経口医薬品になりやすい化合物の化学特性を Rule of five としてまとめ上げた1見ての通り5の倍数に. Archie Battersbees parents have lost a bid to challenge a High Court ruling that denied his transfer to a hospice at the Court of Appeal.

Lecture 12 Concept 08 Lipinski S Rule S Of 5 Youtube
57员环最容易进行关环反应 34员环由于环张力比较大所以闭环速度比较慢 811员环是最难形成的 12员环以上的关环反应由于熵效应的因素比较难形成相对于分子内闭环反应分子间的反应更容易进行.

. The rule was formulated by Christopher A. Christin Lipinski at her home on July 21 2022 in Peoria. It creates a new class of hearing aids that dont require a medical exam a prescription and other.
They include sustaining proliferative signaling evading growth suppressors resisting cell death enabling replicative immortality inducing. The Rule of 5 takes on numeric values from 0 to 4 as a measure of the compounds potential absorption liability. The original Rule of 5 is widely considered to be an important development in modern drug discovery Lipinski et al.
The hallmarks of cancer comprise six biological capabilities acquired during the multistep development of human tumors. 概要 加入喹啉醋酸铅等抑制剂来降低催化剂的活性并且载有碳酸钙的Pd催化剂被称为Lindlar催化剂 由于使用该催化剂进行催化氢化反应的话炔烃的活性比烯烃高炔烃可以很稳定的吸附在催化剂的表面所以Lindlar催化还原炔烃的反应能够停留在烯烃产物并且形成Z-烯烃 基本文献 Lindlar. The Lipinski Rule of Five Lipinski et al 2001 which is so often misused and misunderstood was originally conceived to aid the development of orally bioavailable drugs and was not designed to guide the medicinal chemistry development of all small-molecule drugsOral administration is a desirable objective for the treatment of a number of cancers but is by no.
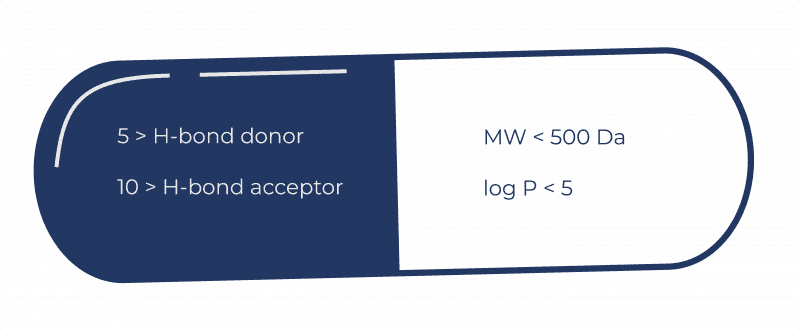
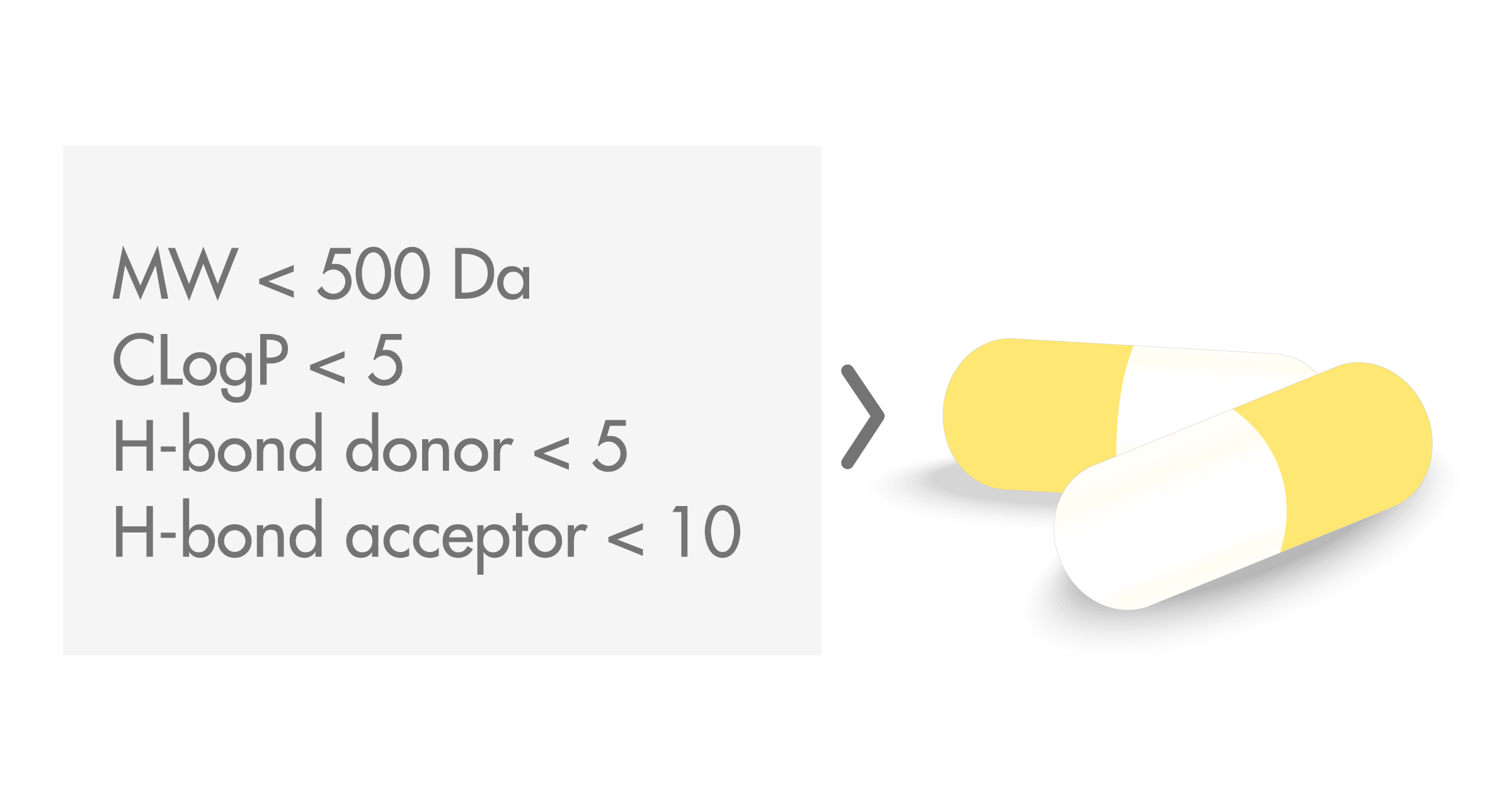
As such the Rule of 5 is a useful computational filter in drug candidate screening. The rule announced Tuesday by the Food and Drug Administration goes into effect this fall. In the discovery setting the rule of 5 predicts that poor absorption or permeation is more likely when there are more than 5 H-bond donors 10 H-bond acceptors the molecular weight MWT is greater than 500 and the.
Lipinskis rule of five also known as Pfizers rule of five or simply the rule of five RO5 is a rule of thumb to evaluate druglikeness or determine if a chemical compound with a certain pharmacological or biological activity has chemical properties and physical properties that would make it a likely orally active drug in humans. Experimental and computational approaches to estimate solubility and permeability in discovery and development settings are described. Lipinski contracted necrotizing fasciitis a rare bacterial infection in January of 2018 and spent 25 months in the Arizona Burn Center.
The hallmarks constitute an organizing principle for rationalizing the complexities of neoplastic disease. The family had sought permission to challenge a ruling.
Pharma Jargon Lipinski S Rule Of Five Pocketful Of Lint
The Rule Of 5 Two Decades Later Sygnature Discovery
No comments for "Lipinski Rule of 5"
Post a Comment